|
SERWIS ELEKTRONICZNY - RADIOELEKTRYKA SOSNOWIEC POLSKA |
|
NIEZALEŻNA DZIAŁALNOŚĆ BADAWCZO - NAUKOWA KLIKNIJ NA OPIS DOKUMENTU |
|
|
|
Threats
Hydrogen cyanide in
cigarettes -
tobacco pipe (highly
toxic) |
|
|
|
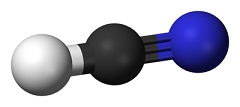
Hydrogen cyanide, HCN
- a chemical compound made of hydrogen, carbon and nitrogen, which
is colorless, volatile and highly poisonous liquid with an odor of
bitter almonds (which however, may be detectable by some
individuals).
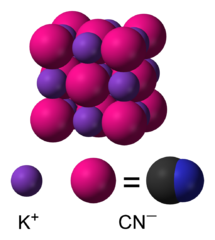
With water forms a weak acid hydrocyanic (prussic acid), which are
called cyanide salts.
He found application in the synthesis and chemical analysis, and as
a pesticide.
During the Second World War was used as an insecticide Zyklon B to
the poisoning of German prisoners of concentration camps.
This includes ogólnotrujących it to chemical agents and is added to the list
3. Convention on the Prohibition of Chemical Weapons. |
|
Historically, industrial
scale cyanide obtained industrially by acidification
cyanide (e.g.. NaCN or Ca (CN) 2).
Currently, there are three industrial processes for obtaining
hydrogen cyanide - Andrussow process, BMA process and the Shawinigan
process.
However, with approx. 25% of the world production of hydrogen
cyanide corresponds to the Sohio process wherein HCN is formed as a
byproduct of the preparation of acrylonitrile from propylene and
ammonia.
The most important industrial production of hydrogen cyanide by the process in
which the exothermic reaction of methane with ammonia, the presence
of oxygen from the air: |
|
|
|
Hydrogen cyanide BMA
process is also produced by the BMA.
Here comes to the reaction of methane with ammonia at high temperature, also
making use of a platinum catalyst: |
|
Properties Hydrogen cyanide
is a colorless liquid with a strong odor of bitter almonds -
perceptibility threshold is 0,002-0,005 mg / dm air.
Some
people can not sense it, however.
It shows high volatility.
Freely
miscible with water and alcohols.
On the
air burns a blue flame.
The vapor form explosive mixtures with air (in the range 6-41% by
volume. Cyjanowodory acid is very weak (pKa = 9.36). Depending on
the conditions can polymerize - oligomers thereof include
aminomalononitryl (trimer), and diaminomalononitryl (tetramer).
Hydrogen cyanide
oxidized during heating (300-650 ° C) in air to form a cyanic acid and minor
amounts of cyan: |
|
|
|
The application is used for disinfection and pest control rooms, for example. Ships or warehouses. The salt form is used in the metallurgical industry, water treatment and classical chemical analysis (CN- ions strongly complexing transition metals). Hydrogen cyanide is used primarily in the production of methyl methacrylate in the plastics industry and in the synthesis for the preparation of potassium cyanide and sodium adiponitrile, methionine, cyanuric chloride, cyanogen, nitrilotriacetic acid and certain of triazine pesticides. It is also used to carry out death penalties in the gas chamber. During the Second World War was used by the Germans under the trade name Zyklon B in mass executions in concentration camps. During World War I attempted to use it as a toxic chemical agent, but without any particular success. Studies on its use for this purpose were conducted in 1915 and by the end of this year, was placed in a mixture of chloroform, arsenic trichloride and tin chloride (IV) in the French missiles No. 4. It was first used in combat operations in July 1916 year. His military markings at the time were Forestite and CK. It is now included on list 3. Convention on the Prohibition of Chemical Weapons. It is highly toxic (lethal dose for a human weighing about 60 kg is approx. 50-60 mg). Hydrogen cyanide is readily absorbed through the lungs, skin and gastrointestinal tract where dissociates and the resulting cyanide ion CN- easily associated with Fe3 + ions and strongly inhibits the enzyme cytochrome oxidase. Has the effect of blocking the transfer of oxygen from the oxyhemoglobin tissues (venous cyanide poisoning has a bright red color, which is normally characteristic of arterial blood). Blocking respiratory enzymes is reversible, which is used in the treatment of victims - CN- have significant affinity for methemoglobin, so poisoned administered isoamyl nitrite or sodium nitrite, to cause methemoglobinemia and restoring transportation of oxygen to the cells. |
|
|